what do they do to u 235 to make it usable
Enriched uranium is a type of uranium in which the pct limerick of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U with 99.2739–99.2752% natural abundance), uranium-235 (235U, 0.7198–0.7202%), and uranium-234 (234U, 0.0050–0.0059%).[one] 235U is the only nuclide existing in nature (in whatever appreciable amount) that is fissile with thermal neutrons.[ii]
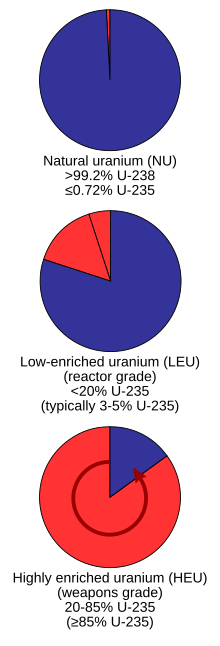
Proportions of uranium-238 (blue) and uranium-235 (red) found naturally versus enriched grades
Enriched uranium is a critical component for both civil nuclear ability generation and military nuclear weapons. The International Atomic Energy Bureau attempts to monitor and control enriched uranium supplies and processes in its efforts to ensure nuclear power generation safety and adjourn nuclear weapons proliferation.
There are about 2,000 tonnes of highly enriched uranium in the earth,[3] produced mostly for nuclear ability, nuclear weapons, naval propulsion, and smaller quantities for inquiry reactors.
The 238U remaining afterward enrichment is known as depleted uranium (DU), and is considerably less radioactive than even natural uranium, though still very dense and extremely hazardous in granulated form – such granules are a natural by-product of the shearing activity that makes information technology useful for armor-penetrating weapons. Despite being mildly radioactive, depleted uranium is also an constructive radiation shielding material.
Grades [edit]
Uranium as information technology is taken straight from the Earth is not suitable every bit fuel for most nuclear reactors and requires additional processes to make it usable (CANDU design is a notable exception). Uranium is mined either underground or in an open pit depending on the depth at which information technology is found. After the uranium ore is mined, it must go through a milling process to extract the uranium from the ore.
This is accomplished past a combination of chemical processes with the stop product existence concentrated uranium oxide, which is known as "yellowcake", contains roughly 80% uranium whereas the original ore typically contains as little every bit 0.1% uranium.[4]
After the milling process is consummate, the uranium must next undergo a process of conversion, "to either uranium dioxide, which can be used as the fuel for those types of reactors that do not crave enriched uranium, or into uranium hexafluoride, which can be enriched to produce fuel for the bulk of types of reactors".[5] Naturally-occurring uranium is made of a mixture of 235U and 238U. The 235U is fissile, meaning information technology is hands separate with neutrons while the residual is 238U, but in nature, more than 99% of the extracted ore is 238U. Most nuclear reactors require enriched uranium, which is uranium with higher concentrations of 235U ranging between three.5% and 4.v% (although a few reactor designs using a graphite or heavy h2o moderator, such every bit the RBMK and CANDU, are capable of operating with natural uranium as fuel). At that place are two commercial enrichment processes: gaseous improvidence and gas centrifugation. Both enrichment processes involve the use of uranium hexafluoride and produce enriched uranium oxide.

A pulsate of yellowcake (a mixture of uranium precipitates)
Reprocessed uranium (RepU) [edit]
Reprocessed uranium (RepU) is a production of nuclear fuel cycles involving nuclear reprocessing of spent fuel. RepU recovered from light h2o reactor (LWR) spent fuel typically contains slightly more than 235U than natural uranium, and therefore could be used to fuel reactors that customarily use natural uranium equally fuel, such as CANDU reactors. It also contains the undesirable isotope uranium-236, which undergoes neutron capture, wasting neutrons (and requiring higher 235U enrichment) and creating neptunium-237, which would be i of the more mobile and troublesome radionuclides in deep geological repository disposal of nuclear waste.
Low-enriched uranium (LEU) [edit]
Low-enriched uranium (LEU) has a lower than 20% concentration of 235U; for instance, in commercial LWR, the near prevalent ability reactors in the world, uranium is enriched to three to 5% 235U. High-assay LEU (HALEU) is enriched from five–20%.[6] Fresh LEU used in enquiry reactors is usually enriched 12 to 19.75% 235U, the latter concentration is used to supplant HEU fuels when converting to LEU.[7]
Highly enriched uranium (HEU) [edit]

A billet of highly enriched uranium metal
Highly enriched uranium (HEU) has a xx% or college concentration of 235U. The fissile uranium in nuclear weapon primaries usually contains 85% or more than of 235U known as weapons-grade, though theoretically for an implosion design, a minimum of 20% could be sufficient (chosen weapon-usable) although it would crave hundreds of kilograms of textile and "would not be practical to blueprint";[8] [9] even lower enrichment is hypothetically possible, only equally the enrichment per centum decreases the critical mass for unmoderated fast neutrons rapidly increases, with for example, an infinite mass of 5.4% 235U being required.[8] For criticality experiments, enrichment of uranium to over 97% has been accomplished.[10]
The very get-go uranium bomb, Footling Boy, dropped by the United states on Hiroshima in 1945, used 64 kilograms of 80% enriched uranium. Wrapping the weapon'southward fissile core in a neutron reflector (which is standard on all nuclear explosives) tin can dramatically reduce the disquisitional mass. Because the core was surrounded by a skillful neutron reflector, at explosion it comprised almost two.five disquisitional masses. Neutron reflectors, compressing the fissile core via implosion, fusion boosting, and "tamping", which slows the expansion of the fissioning core with inertia, allow nuclear weapon designs that use less than what would be one blank-sphere critical mass at normal density. The presence of too much of the 238U isotope inhibits the runaway nuclear chain reaction that is responsible for the weapon's ability. The critical mass for 85% highly enriched uranium is nigh 50 kilograms (110 lb), which at normal density would be a sphere near 17 centimetres (6.seven in) in diameter.
Later U.s.a. nuclear weapons unremarkably use plutonium-239 in the principal stage, simply the jacket or tamper secondary stage, which is compressed by the primary nuclear explosion ofttimes uses HEU with enrichment betwixt 40% and 80%[xi] along with the fusion fuel lithium deuteride. For the secondary of a large nuclear weapon, the higher critical mass of less-enriched uranium tin be an advantage as information technology allows the core at explosion time to incorporate a larger amount of fuel. The 238U is not said to be fissile but however is fissionable past fast neutrons (>two MeV) such as the ones produced during D-T fusion.
HEU is besides used in fast neutron reactors, whose cores crave about 20% or more of fissile material, as well every bit in naval reactors, where it ofttimes contains at least 50% 235U, but typically does not exceed 90%. The Fermi-1 commercial fast reactor prototype used HEU with 26.v% 235U. Significant quantities of HEU are used in the production of medical isotopes, for example molybdenum-99 for technetium-99m generators.[12]
Enrichment methods [edit]
Isotope separation is difficult because two isotopes of the aforementioned element have virtually identical chemical properties, and can simply exist separated gradually using small mass differences. (235U is merely ane.26% lighter than 238U). This problem is compounded considering uranium is rarely separated in its atomic form, just instead as a compound (235UF6 is simply 0.852% lighter than 238UFhalf-dozen). A cascade of identical stages produces successively college concentrations of 235U. Each stage passes a slightly more full-bodied product to the next stage and returns a slightly less concentrated rest to the previous phase.
There are currently 2 generic commercial methods employed internationally for enrichment: gaseous diffusion (referred to as first generation) and gas centrifuge (second generation), which consumes just 2% to 2.5%[13] every bit much free energy as gaseous diffusion (at least a "cistron of 20" more efficient).[14] Some piece of work is being done that would utilize nuclear resonance; however at that place is no reliable bear witness that whatever nuclear resonance processes have been scaled up to production.
Improvidence techniques [edit]
Gaseous diffusion [edit]

Gaseous diffusion uses semi-permeable membranes to split enriched uranium
Gaseous improvidence is a technology used to produce enriched uranium by forcing gaseous uranium hexafluoride (hex) through semi-permeable membranes. This produces a slight separation between the molecules containing 235U and 238U. Throughout the Cold State of war, gaseous diffusion played a major role as a uranium enrichment technique, and as of 2008 accounted for about 33% of enriched uranium product,[15] but in 2011 was deemed an obsolete technology that is steadily being replaced past the later generations of technology every bit the improvidence plants accomplish their ends-of-life.[16] In 2013, the Paducah facility in the The states ceased operating, it was the final commercial 235U gaseous diffusion institute in the globe.[17]
Thermal diffusion [edit]
Thermal diffusion uses the transfer of oestrus beyond a thin liquid or gas to reach isotope separation. The process exploits the fact that the lighter 235U gas molecules volition diffuse toward a hot surface, and the heavier 238U gas molecules will lengthened toward a common cold surface. The S-50 institute at Oak Ridge, Tennessee was used during Earth State of war II to gear up feed cloth for the EMIS process. It was abandoned in favor of gaseous improvidence.
Centrifuge techniques [edit]
Gas centrifuge [edit]

A cascade of gas centrifuges at a U.S. enrichment plant
The gas centrifuge process uses a large number of rotating cylinders in serial and parallel formations. Each cylinder's rotation creates a strong centripetal force so that the heavier gas molecules containing 238U move tangentially toward the exterior of the cylinder and the lighter gas molecules rich in 235U collect closer to the middle. Information technology requires much less energy to achieve the aforementioned separation than the older gaseous improvidence process, which it has largely replaced and so is the current method of choice and is termed second generation. Information technology has a separation gene per stage of 1.3 relative to gaseous diffusion of 1.005,[xv] which translates to about i-fiftieth of the energy requirements. Gas centrifuge techniques produce close to 100% of the world'south enriched uranium.
Zippe centrifuge [edit]

Diagram of the principles of a Zippe-type gas centrifuge with U-238 represented in dark blue and U-235 represented in light blue
The Zippe-type centrifuge is an improvement on the standard gas centrifuge, the primary difference being the use of heat. The lesser of the rotating cylinder is heated, producing convection currents that move the 235U upwards the cylinder, where it can be collected by scoops. This improved centrifuge blueprint is used commercially by Urenco to produce nuclear fuel and was used by Pakistan in their nuclear weapons program.
Laser techniques [edit]
Laser processes hope lower energy inputs, lower capital costs and lower tails assays, hence significant economical advantages. Several laser processes have been investigated or are under development. Separation of isotopes by laser excitation (SILEX) is well developed and is licensed for commercial operation every bit of 2012.
Atomic vapor laser isotope separation (AVLIS) [edit]
Atomic vapor light amplification by stimulated emission of radiation isotope separation employs particularly tuned lasers[18] to split up isotopes of uranium using selective ionization of hyperfine transitions. The technique uses lasers tuned to frequencies that ionize 235U atoms and no others. The positively charged 235U ions are so attracted to a negatively charged plate and collected.
Molecular light amplification by stimulated emission of radiation isotope separation (MLIS) [edit]
Molecular laser isotope separation uses an infrared laser directed at UF6, exciting molecules that contain a 235U cantlet. A second laser frees a fluorine atom, leaving uranium pentafluoride, which and so precipitates out of the gas.
Separation of isotopes by laser excitation (SILEX) [edit]
Separation of isotopes by laser excitation is an Australian development that likewise uses UFhalf dozen. Afterwards a protracted development procedure involving U.South. enrichment visitor USEC acquiring and then relinquishing commercialization rights to the technology, GE Hitachi Nuclear Energy (GEH) signed a commercialization agreement with Silex Systems in 2006.[xix] GEH has since built a demonstration test loop and announced plans to build an initial commercial facility.[xx] Details of the process are classified and restricted past intergovernmental agreements betwixt United states of america, Commonwealth of australia, and the commercial entities. SILEX has been projected to exist an order of magnitude more efficient than existing production techniques only again, the exact figure is classified.[15] In August, 2011 Global Laser Enrichment, a subsidiary of GEH, applied to the U.S. Nuclear Regulatory Commission (NRC) for a permit to build a commercial institute.[21] In September 2012, the NRC issued a license for GEH to build and operate a commercial SILEX enrichment institute, although the company had not all the same decided whether the project would be assisting enough to brainstorm construction, and despite concerns that the technology could contribute to nuclear proliferation.[22]
Other techniques [edit]
Aerodynamic processes [edit]

Schematic diagram of an aerodynamic nozzle. Many thousands of these small foils would be combined in an enrichment unit.

The X-ray based LIGA manufacturing process was originally adult at the Forschungszentrum Karlsruhe, Germany, to produce nozzles for isotope enrichment.[23]
Aerodynamic enrichment processes include the Becker jet nozzle techniques developed by E. W. Becker and associates using the LIGA process and the vortex tube separation process. These aerodynamic separation processes depend upon diffusion driven by pressure level gradients, as does the gas centrifuge. They in general have the disadvantage of requiring complex systems of cascading of individual separating elements to minimize energy consumption. In effect, aerodynamic processes can be considered as non-rotating centrifuges. Enhancement of the centrifugal forces is accomplished by dilution of UF6 with hydrogen or helium as a carrier gas achieving a much college menses velocity for the gas than could exist obtained using pure uranium hexafluoride. The Uranium Enrichment Corporation of Due south Africa (UCOR) developed and deployed the continuous Helikon vortex separation cascade for high production rate low-enrichment and the substantially dissimilar semi-batch Pelsakon low product rate high enrichment cascade both using a particular vortex tube separator design, and both embodied in industrial institute.[24] A sit-in constitute was built in Brazil by NUCLEI, a consortium led by Industrias Nucleares do Brasil that used the separation nozzle process. Nevertheless all methods accept high energy consumption and substantial requirements for removal of waste heat; none is currently yet in use.
Electromagnetic isotope separation [edit]

Schematic diagram of uranium isotope separation in a calutron shows how a stiff magnetic field is used to redirect a stream of uranium ions to a target, resulting in a college concentration of uranium-235 (represented here in nighttime blue) in the inner fringes of the stream.
In the electromagnetic isotope separation procedure (EMIS), metallic uranium is get-go vaporized, and so ionized to positively charged ions. The cations are then accelerated and afterwards deflected by magnetic fields onto their respective collection targets. A production-calibration mass spectrometer named the Calutron was developed during World State of war Two that provided some of the 235U used for the Little Boy nuclear bomb, which was dropped over Hiroshima in 1945. Properly the term 'Calutron' applies to a multistage device arranged in a large oval around a powerful electromagnet. Electromagnetic isotope separation has been largely abandoned in favour of more effective methods.
Chemical methods [edit]
1 chemic process has been demonstrated to pilot plant stage only not used for production. The French CHEMEX process exploited a very slight difference in the two isotopes' propensity to change valency in oxidation/reduction, using immiscible aqueous and organic phases. An ion-substitution process was developed past the Asahi Chemical Company in Japan that applies similar chemistry but effects separation on a proprietary resin ion-exchange cavalcade.
Plasma separation [edit]
Plasma separation process (PSP) describes a technique that makes use of superconducting magnets and plasma physics. In this process, the principle of ion cyclotron resonance is used to selectively energize the 235U isotope in a plasma containing a mix of ions. France adult its own version of PSP, which it called RCI. Funding for RCI was drastically reduced in 1986, and the plan was suspended effectually 1990, although RCI is all the same used for stable isotope separation.
Separative work unit [edit]
"Separative piece of work" – the amount of separation done past an enrichment process – is a function of the concentrations of the feedstock, the enriched output, and the depleted tailings; and is expressed in units that are and then calculated as to be proportional to the total input (energy / machine operation time) and to the mass candy. Separative work is not energy. The same amount of separative work volition require different amounts of free energy depending on the efficiency of the separation engineering science. Separative work is measured in Separative work units SWU, kg SW, or kg UTA (from the German Urantrennarbeit – literally uranium separation work)
- 1 SWU = 1 kg SW = 1 kg UTA
- 1 kSWU = ane tSW = 1 t UTA
- 1 MSWU = 1 ktSW = 1 kt UTA
Cost issues [edit]
In add-on to the separative work units provided by an enrichment facility, the other important parameter to exist considered is the mass of natural uranium (NU) that is needed to yield a desired mass of enriched uranium. As with the number of SWUs, the corporeality of feed fabric required will also depend on the level of enrichment desired and upon the amount of 235U that ends up in the depleted uranium. However, unlike the number of SWUs required during enrichment, which increases with decreasing levels of 235U in the depleted stream, the amount of NU needed will decrease with decreasing levels of 235U that finish up in the DU.
For instance, in the enrichment of LEU for utilize in a lite water reactor it is typical for the enriched stream to incorporate three.6% 235U (every bit compared to 0.vii% in NU) while the depleted stream contains 0.2% to 0.3% 235U. In order to produce one kilogram of this LEU information technology would crave approximately viii kilograms of NU and 4.five SWU if the DU stream was allowed to have 0.3% 235U. On the other hand, if the depleted stream had only 0.2% 235U, then it would require simply 6.seven kilograms of NU, but nearly 5.7 SWU of enrichment. Because the amount of NU required and the number of SWUs required during enrichment change in reverse directions, if NU is cheap and enrichment services are more expensive, then the operators will typically choose to allow more 235U to exist left in the DU stream whereas if NU is more than expensive and enrichment is less so, then they would choose the opposite.
When converting uranium (hexafluoride, hex for brusque) to metallic, .iii% is lost during manufacturing.[25] [26]
Downblending [edit]
The opposite of enriching is downblending; surplus HEU tin can be downblended to LEU to go far suitable for use in commercial nuclear fuel.
The HEU feedstock can incorporate unwanted uranium isotopes: 234U is a minor isotope contained in natural uranium (primarily every bit a production of alpha decay of 238
U - because the half-life of 238
U is much larger than that of 234
U, it'll be produced and destroyed at the aforementioned charge per unit in a abiding steady state equilibrium, bringing any sample with sufficient 238
U content to a stable ratio of 234
U to 238
U over long enough timescales); during the enrichment process, its concentration increases but remains well beneath 1%. High concentrations of 236U are a byproduct from irradiation in a reactor and may be contained in the HEU, depending on its manufacturing history. 236
U is produced primarily when 235
U absorbs a neutron and does non fission. The production of 236
U is thus unavoidable in any thermal neutron reactor with 235
U fuel. HEU reprocessed from nuclear weapons material production reactors (with an 235U assay of approx. 50%) may comprise 236U concentrations every bit high as 25%, resulting in concentrations of approximately 1.5% in the blended LEU product. 236U is a neutron poison; therefore the actual 235U concentration in the LEU production must exist raised accordingly to compensate for the presence of 236U. While 234
U also absorbs neutrons, it is a fertile material that is turned into fissile 235
U upon neutron assimilation. If 236
U absorbs a neutron, the resulting short-lived 237
U beta decays to 237
Np, which is not usable in thermal neutron reactors but tin be chemically separated from spent fuel to be disposed of as waste matter or to be transmutated into 238
Pu (for utilize in nuclear batteries) in special reactors.
The blendstock tin be NU, or DU, however depending on feedstock quality, SEU at typically one.5 wt% 235U may be used as a blendstock to dilute the unwanted byproducts that may be contained in the HEU feed. Concentrations of these isotopes in the LEU product in some cases could exceed ASTM specifications for nuclear fuel, if NU, or DU were used. So, the HEU downblending generally cannot contribute to the waste management trouble posed past the existing large stockpiles of depleted uranium. At nowadays, 95 pct of the world'southward stocks of depleted uranium remain in secure storage.[ citation needed ]
A major downblending undertaking called the Megatons to Megawatts Program converts ex-Soviet weapons-course HEU to fuel for U.S. commercial power reactors. From 1995 through mid-2005, 250 tonnes of high-enriched uranium (enough for 10,000 warheads) was recycled into low-enriched-uranium. The goal is to recycle 500 tonnes by 2013. The decommissioning plan of Russian nuclear warheads accounted for about thirteen% of total earth requirement for enriched uranium leading up to 2008.[15]
The United States Enrichment Corporation has been involved in the disposition of a portion of the 174.3 tonnes of highly enriched uranium (HEU) that the U.S. government declared equally surplus military material in 1996. Through the U.S. HEU Downblending Program, this HEU material, taken primarily from dismantled U.S. nuclear warheads, was recycled into depression-enriched uranium (LEU) fuel, used past nuclear power plants to generate electricity.[27] [28]
Global enrichment facilities [edit]
The post-obit countries are known to operate enrichment facilities: Argentine republic, Brazil, Communist china, France, Germany, India, Islamic republic of iran, Japan, kingdom of the netherlands, Democratic people's republic of korea, Pakistan, Russia, the United Kingdom, and the U.s.a..[29] [30] Belgium, Iran, Italia, and Espana hold an investment interest in the French Eurodif enrichment plant, with Iran's holding entitling it to 10% of the enriched uranium output. Countries that had enrichment programs in the past include Great socialist people's libyan arab jamahiriya and South Africa, although Libya'south facility was never operational.[31] Commonwealth of australia has developed a light amplification by stimulated emission of radiation enrichment procedure known as SILEX, which it intends to pursue through financial investment in a U.South. commercial venture past General Electric.[32] Information technology has also been claimed that Israel has a uranium enrichment program housed at the Negev Nuclear Enquiry Center site near Dimona.[33]
Codename [edit]
During the Manhattan Projection, weapons-grade highly enriched uranium was given the codename oralloy, a shortened version of Oak Ridge blend, after the location of the plants where the uranium was enriched.[34] The term oralloy is still occasionally used to refer to enriched uranium.
See also [edit]
- List of laser articles
- MOX fuel
- Nuclear fuel banking concern
- Orano
- Uranium market
- Uranium mining
References [edit]
- ^ "Uranium Isotopes". GlobalSecurity.org. Retrieved 5 February 2020.
- ^ OECD Nuclear Energy Agency (2003). Nuclear Free energy Today. OECD Publishing. p. 25. ISBN9789264103283.
- ^ Thomas B. Cochran (Natural Resources Defense Council) (12 June 1997). "Safeguarding Nuclear Weapon-Usable Materials in Russia" (PDF). Proceedings of international forum on illegal nuclear traffic. Archived from the original (PDF) on 22 July 2012.
- ^ Nuclear Fuel Bicycle Overview, Uranium milling. World Nuclear Clan, update April 2021
- ^ "Radiological Sources of Potential Exposure and/or Contamination". U.S. Ground forces Center for Wellness Promotion and Preventive Medicine. June 1999. p. 27. Retrieved one July 2019.
- ^ Herczeg, John Due west. (28 March 2019). "High-assay depression enriched uranium" (PDF). energy.gov.
- ^ Alexander Glaser (6 November 2005). "About the Enrichment Limit for Enquiry Reactor Conversion : Why xx%?" (PDF). Princeton Academy. Retrieved 18 April 2014.
- ^ a b Forsberg, C. Due west.; Hopper, C. G.; Richter, J. L.; Vantine, H. C. (March 1998). "Definition of Weapons-Usable Uranium-233" (PDF). ORNL/TM-13517. Oak Ridge National Laboratories. Archived from the original (PDF) on ii Nov 2013. Retrieved 30 October 2013.
- ^ Sublette, Carey (4 October 1996). "Nuclear Weapons FAQ, Section iv.one.7.1: Nuclear Design Principles – Highly Enriched Uranium". Nuclear Weapons FAQ . Retrieved 2 October 2010.
- ^ Mosteller, R.D. (1994). "Detailed Reanalysis of a Benchmark Critical Experiment: Water-Reflected Enriched-Uranium Sphere" (PDF). Los Alamos Technical Paper (LA–UR–93–4097): 2. doi:10.2172/10120434. Retrieved 19 December 2007.
The enrichment of the pin and of one of the hemispheres was 97.67 w/o, while the enrichment of the other hemisphere was 97.68 w/o.
- ^ "Nuclear Weapons FAQ". Retrieved 26 Jan 2013.
- ^ Frank N. Von Hippel; Laura H. Kahn (December 2006). "Feasibility of Eliminating the Use of Highly Enriched Uranium in the Production of Medical Radioisotopes". Science & Global Security. 14 (two & 3): 151–162. Bibcode:2006S&GS...14..151V. doi:10.1080/08929880600993071. S2CID 122507063.
- ^ "Uranium Enrichment". earth-nuclear.org.
- ^ Economical Perspective for Uranium Enrichment (PDF),
The throughput per centrifuge unit is very small compared to that of a diffusion unit so small, in fact, that it is non compensated past the higher enrichment per unit. To produce the same amount of reactor-course fuel requires a considerably larger number (approximately l,000 to 500,000) of centrifuge units than diffusion units. This disadvantage, notwithstanding, is outweighed by the considerably lower (by a cistron of 20) energy consumption per SWU for the gas centrifuge
- ^ a b c d "Lodge Partners Mid-Cap Conference eleven Apr 2008" (PDF). Silex Ltd. 11 April 2008.
- ^ Rod Adams (24 May 2011). "McConnell asks DOE to keep using 60-year-erstwhile enrichment plant to save jobs". Atomic Insights. Archived from the original on 28 Jan 2013. Retrieved 26 Jan 2013.
- ^ "Paducah enrichment plant to exist closed. The 1950s facility is the last remaining gaseous diffusion uranium enrichment found in the world.".
- ^ F. J. Duarte and L.W. Hillman (Eds.), Dye Light amplification by stimulated emission of radiation Principles (Bookish, New York, 1990) Chapter 9.
- ^ "GE Signs Agreement With Silex Systems of Australia To Develop Uranium Enrichment Technology" (Printing release). GE Energy. 22 May 2006. Archived from the original on 14 June 2006.
- ^ "GE Hitachi Nuclear Energy Selects Wilmington, N.C. as Site for Potential Commercial Uranium Enrichment Facility". Business Wire. 30 April 2008. Retrieved 30 September 2012.
- ^ Wide, William J. (20 August 2011). "Laser Advances in Nuclear Fuel Stir Terror Fear". The New York Times . Retrieved 21 Baronial 2011.
- ^ "Uranium Plant Using Laser Technology Wins U.S. Approval". The New York Times. September 2012.
- ^ Becker, Due east. W.; Ehrfeld, Due west.; Münchmeyer, D.; Betz, H.; Heuberger, A.; Pongratz, S.; Glashauser, W.; Michel, H. J.; Siemens, R. (1982). "Production of Separation-Nozzle Systems for Uranium Enrichment by a Combination of X-Ray Lithography and Galvanoplastics". Naturwissenschaften. 69 (11): 520–523. Bibcode:1982NW.....69..520B. doi:ten.1007/BF00463495. S2CID 44245091.
- ^ Smith, Michael; Jackson A G M (2000). "Dr". Southward African Institution of Chemical Engineers – Conference 2000: 280–289.
- ^ Balakrishnan, M. R. (1971). "Economic science of blending, a instance report" (PDF). Bombay, India: Government of Bharat, Atomic Energy Commission. p. 6. Retrieved 7 November 2021.
- ^ US Atomic Energy Commission (January 1961). "Costs of nuclear power". Washington DC: Function of Technical Services, Dept of Commerce. p. 29. Retrieved 7 November 2021.
- ^ "Condition Report: USEC-DOE Megatons to Megawatts Plan". USEC.com. ane May 2000. Archived from the original on six April 2001.
- ^ "Megatons to Megawatts". centrusenergy.com. December 2013.
- ^ Arjun Makhijani; Lois Chalmers; Brice Smith (15 October 2004). Uranium enrichment (PDF). Institute for Free energy and Environmental Research. Retrieved 21 November 2009.
- ^ Australia's uranium - Greenhouse friendly fuel for an energy hungry world (PDF). Standing Committee on Industry and Resource (Report). The Parliament of the Commonwealth of Australia. November 2006. p. 730. Retrieved 3 Apr 2015.
- ^ "Q&A: Uranium enrichment". BBC News. BBC. one September 2006. Retrieved 3 January 2010.
- ^ "Laser enrichment could cutting toll of nuclear ability". The Sydney Forenoon Herald. 26 May 2006.
- ^ "Israel'southward Nuclear Weapons Plan". Nuclear Weapon Annal. ten Dec 1997. Retrieved 7 Oct 2007.
- ^ William Burr (22 December 2015). "Strategic Air Command Declassifies Nuclear Target List from 1950s". nsarchive2.gwu.edu . Retrieved 27 Nov 2020.
Oralloy [Oak Ridge alloy] was a term of art for highly-enriched uranium.
External links [edit]
- Annotated bibliography on enriched uranium from the Alsos Digital Library for Nuclear Problems
- Silex Systems Ltd
- Uranium Enrichment, World Nuclear Association
- Overview and history of U.S. HEU production
- News Resource on Uranium Enrichment
- Nuclear Chemistry-Uranium Enrichment
- A decorated twelvemonth for SWU (a 2008 review of the commercial enrichment marketplace), Nuclear Applied science International, 1 September 2008
- Uranium Enrichment and Nuclear Weapon Proliferation, by Allan S. Krass, Peter Boskma, Boelie Elzen and Wim A. Smit, 296 pp., published for SIPRI by Taylor and Francis Ltd, London, 1983
- Poliakoff, Martyn (2009). "How do yous enrich Uranium?". The Periodic Tabular array of Videos. University of Nottingham.
- Gilinsky, V.; Hoehn, W. (Dec 1969). "The Military Significance of Small Uranium Enrichment Facilities Fed with Depression-Enrichment Uranium (Redacted)". Defence Technical Information Middle. RAND Corporation. Archived from the original on 16 Feb 2016. Retrieved 12 February 2016.
Source: https://en.wikipedia.org/wiki/Enriched_uranium
Postar um comentário for "what do they do to u 235 to make it usable"